Bacteria represent the simplest forms of life, which makes them powerful tools to study the basic concepts of cellular organization. Despite their comparably simple architecture, they have evolved an amazing variety of mechanisms to control the spatial arrangement of cellular components, including localized protein complexes, diverse cytoskeletal structures, and complex signalling cascades. These regulatory systems control a range of essential processes, such as cell growth, cell differentiation, chromosome segregation, and cell division.
Our group investigates the molecular mechanisms underlying the spatiotemporal organization of bacterial cells, with an emphasis on the model bacteria Caulobacter crescentus, Hyphomonas neptunium, and Myxococcus xanthus. In particular, we are focusing on the following topics:
Our group investigates the molecular mechanisms underlying the spatiotemporal organization of bacterial cells, with an emphasis on the model bacteria Caulobacter crescentus, Hyphomonas neptunium, and Myxococcus xanthus. In particular, we are focusing on the following topics:
Chromosome segregation
Regulation of division site placement
Cytokinesis has to be closely coordinated with chromosome replication and segregation to generate viable offspring that contains the full complement of hereditary information. In most bacteria, this process is mediated by a ring-shaped multi-protein complex, called the divisome, which spans all layers of the cell envelope. The basis of the divisome is formed by polymers of the tubulin homologue FtsZ that, directly or indirectly, recruit all other divisome components and, furthermore, play a pivotal active role in the subsequent constriction process. Given its fundamental role in cell division, FtsZ is the primary target of systems involved in the temporal and spatial regulation of cytokinesis.
|
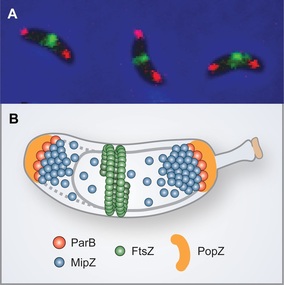
We have previously identified a novel regulatory mechanism that controls the positioning of the division plane in C. crescentus and probably many other alpha-proteobacterial species (Thanbichler & Shapiro, 2006). It is founded on the P-loop ATPase MipZ, which forms a dynamic complex with the DNA partitioning protein ParB at the chromosomal origin of replication. In the predivisional cell, the two copies of the origin/ParB complex are sequestered to opposite cell poles through interaction with the scaffolding protein PopZ. As a consequence, MipZ forms a gradient within the cytoplasm, with its concentration being highest at the tips of the cell and lowest at the cell center. MipZ acts as a direct inhibitor of FtsZ polymerization, thus limiting formation of the division apparatus to the midcell region.
Steady-state protein gradients are a well-known regulatory strategy in eukaryotic cells, usually established by localized protein synthesis followed by subsequent diffusion and protein degradation. However, such diffusional gradients are extremely unstable at the scale of a small prokaryotic cell, suggesting that formation of the MipZ gradient is based on a different principle. We have solved the crystal structures of MipZ in the apo and ATP-bound form and analyzed the mechanism of MipZ localization (Kiekebusch et al., 2012). Our studies showed that MipZ serves as a molecular switch that, similar to small GTPases, uses nucleotide binding and hydrolysis to alternate between two different functional states with distinct interaction networks and diffusion rates. This behavior drives a unique localization cycle, with MipZ molecules oscillating back and forth between the polar ParB complexes and pole-distal regions of the nucleoid. These findings for the first time shed light on the basis of steady-state gradient formation in bacteria and might provide a general mechanistic framework for other gradient-forming systems in both prokaryotic and eukaroytic cells (Kiekebusch & Thanbichler, 2013). |
Division site placement by MipZ. (A) In vivo localization of FtsZ (green) and ParB (red). (B) Schematic showing the compo-nents of the MipZ system.
|
Bactofilins: a new cytoskeletal scaffold
Cytoskeletal elements have a central role in the temporal and spatial organization of cellular processes in bacteria. Among the most widely conserved cytoskeletal proteins are the tubulin homologue FtsZ, the actin homologue MreB, and intermediate filament-like proteins, which perform important roles in cell division, morphogenesis, and cell polarity.
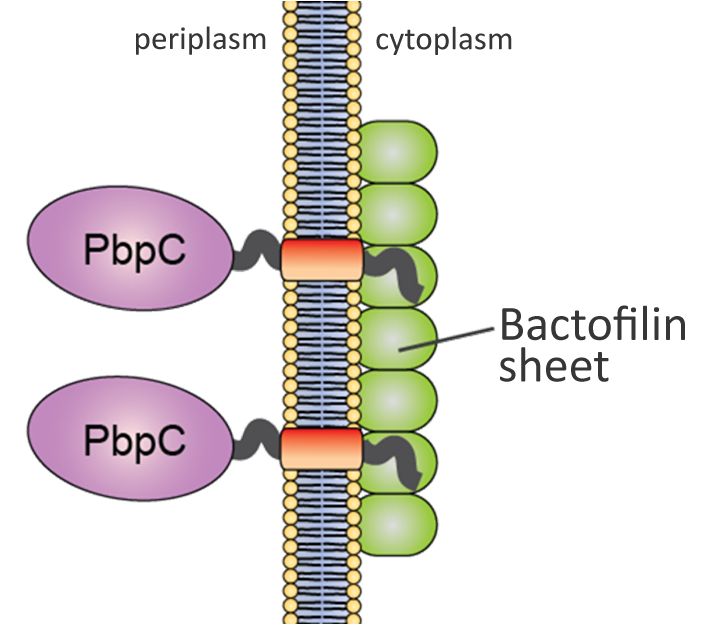
Our group has recently identified a novel, fourth class of cytoskeletal proteins, designated bactofilins, which are widespread among bacteria. Studies in C. crescentus have shown that the two bactofilin paralogues synthesized in this organism (BacA and BacB) interact with each other and assemble into membrane-associated, polymeric sheets that are specifically localized to the old cell pole during defined stages of the cell cycle (Kühn et al., 2010). These structures serve as platforms for the assembly and polar localization of a cell wall biosynthetic enzyme (PbpC) involved in the biogenesis of stalk, an extension of the cell body characteristic of C. crescentus and many of its relatives. In vitro analysis showed that bactofilins polymerize spontaneously and in the absence of nucleotide cofactors, forming long, biochemically inert filament bundles. This behavior is reminiscent of intermediate filaments, even though there is no evolutionary or structural relationship between these two groups of proteins (Lin & Thanbichler, 2013).
Our group has recently identified a novel, fourth class of cytoskeletal proteins, designated bactofilins, which are widespread among bacteria. Studies in C. crescentus have shown that the two bactofilin paralogues synthesized in this organism (BacA and BacB) interact with each other and assemble into membrane-associated, polymeric sheets that are specifically localized to the old cell pole during defined stages of the cell cycle (Kühn et al., 2010). These structures serve as platforms for the assembly and polar localization of a cell wall biosynthetic enzyme (PbpC) involved in the biogenesis of stalk, an extension of the cell body characteristic of C. crescentus and many of its relatives. In vitro analysis showed that bactofilins polymerize spontaneously and in the absence of nucleotide cofactors, forming long, biochemically inert filament bundles. This behavior is reminiscent of intermediate filaments, even though there is no evolutionary or structural relationship between these two groups of proteins (Lin & Thanbichler, 2013).
|
Model of C. crescentus BacAB function. Membrane-associated polymers of the bactofilins BacAB recruit the cell cell wall synthase PbpC through interaction with its N-terminal cytoplasmic tail.
|
Database searches showed that bactofilins are almost universially conserved among bacteria and often present in multiple paralogous copies per species. To clarify the spectrum of functions they can perform, we have set out to study the role of bactofilins in other bacteria. In particular, we are currently focusing on the delta-proteobacterium Myxococcus xanthus, an organism containing four bactofilin homologues. In collaboration with the group of Lotte Søgaard-Andersen, we have shown that three of these proteins (BacNOP) co-polymerize into extended bipolar filaments. These structures, on the one hand, mediate the subpolar localization of a small GTPase controlling the positioning of regulatory proteins involved in cell motility (Bulyha et al., 2013). On the other hand, they contribute to proper chromosome organization and segregation by controlling the localization of a P-loop ATPase involved in chromosome segregation (ParA) and by immobilizing the chromosomal ParB/ origin complexes in the subpolar regions of the cell (Lin et al., 2017). Collectively, these findings suggest that bactofilins serve as multifunctional polymeric scaffolds that ensure the proper arrangement of proteins within bacterial cells. Notably, work by other groups has shown that a bactofilin homolog is critical for cell shape and virulence in the human pathogen Helicobacter pylori, the causative agent of stomach ulcers. Bactofilins thus appear to have a range of critical functions that still await discovery in the future. It could be interesting to harness these structures for the development of synthetic molecular landmarks that enable the specific targeting of cellular components in artificial or heterologous cellular systems.
|
We are currently investigating the biological roles of bactofilin polymers and the mechanisms regulating their positioning and assembly in different model systems. Moreover, we aim at clarifying its mode of interaction with target proteins.
Function of the divisome
The divisome is a complex machinery composed of more than two dozen proteins. Some of its core components, such as FtsZ, are highly conserved among bacteria, whereas others are restricted to certain lineages, adapting divisome function to the specific physiological needs of the host. Despite decades of intensive research, the precise function of most core component and the mechanisms coordinating their activities are still unclear. Previous work on cell division has mainly focused on the model organism Escherichia coli. The essential divisome components identified in this organism are conserved in other bacteria, although their sequences are in some cases highly divergent. We have recently identified additional, new components of the C. crescentus divisome and revealed significant differences in the orchestration of the division process compared to the canonical E. coli system.
|
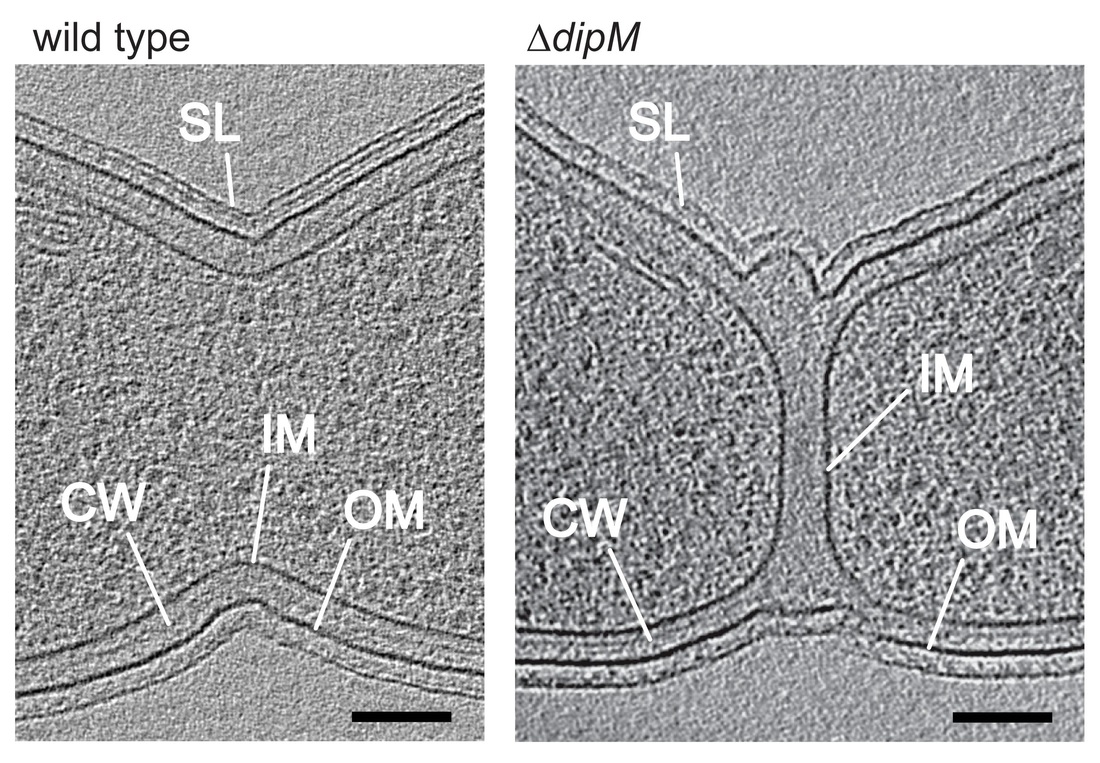
Investigating the machinery involved in cell wall constriction, we discovered in C. crescentus a previously uncharacterized regulatory protein that turned out to be a distant homolog of the known cell division protein FtsN. While FtsN was previously thought to be present only in E. coli and closely related species, we found that equivalent proteins are in fact present in all proteobacterial species (Möll & Thanbichler, 2009). Importantly, we were able to show that the cell wall-binding (SPOR) domain that is characteristic of FtsN, and widespread among many other proteins involved in cell wall biogenesis, acts as a general module mediating the recruitment of cell wall biosynthetic factors to the bacterial division site. Screening for interaction partners of C. crescentus FtsN, we have revealed a second factor with a key role in the coordination of the cell wall remodeling process, DipM (Möll et al., 2010). Interestingly, the function of DipM relies on a cell wall hydrolytic domain that has been rendered inactive by mutations in several catalytic residues. A similar situation has been seen in E. coli, where an unrelated protein bearing the same inactive hydrolase domain was shown to be part of a regulatory pathway coordinating cell wall constriction. However, this pathway is only conserved in close relatives of E. coli, indicating that the regulatory mechanisms at work in other bacteria must be different.
|
Function of DipM. Shown are virtual sections through electron cryo-tomograms of C. crescentus wild-type and dipM mutant cells. In the mutant, invagination of the inner and outer layers of the cell envelope are uncoupled (IM: inner membrane, CW: cell wall, OM: outer membrane, SL: surface layer).
|
To elucidate the regulation of cell wall constriction in C. crescentus, we have recently initiated a comprehensive analysis of the numerous enzymes involved in peptidoglycan synthesis and degradation in this species. These studies have revealed that several additional peptidoglycan synthases and hydrolases of C. crescentus are recruited to the division site. However, in most cases, these proteins are not essential for the division process, because their loss can be compensated by various functionally equivalent proteins that are not specifically associated with the division apparatus (Strobel et al., 2014; Zielinska et al., 2017). Thus, the different growth modes of bacterial cells are mediated by a highly redundant set of enzymes with broad and overlapping functionality. We have now set out to determine how the activities of the different players are coordinated such as to ensure proper cell shape and cell wall integrity.
Apart from our work on peptidoglycan remodeling enzymes, we are currently studying other newly identified cell division proteins to clarify their biological function and their mode of interaction with other divisome components.
Apart from our work on peptidoglycan remodeling enzymes, we are currently studying other newly identified cell division proteins to clarify their biological function and their mode of interaction with other divisome components.
Hyphomonas neptunium
To date, cell growth, chromosome segregation, and cell division have, by and large, only been studied in bacteria that divide by binary fission. To expand our knowledge of the mechanisms that govern the spatiotemporal organization of bacterial cells, we have recently established the stalked budding bacterium Hyphomonas neptunium as a new model system in our group.
|
H. neptunium is a close relative of C. crescentus that proliferates by a unique budding mechanisms in which daughter cells are released from the tip of a long stalk emanating from the mother cell body. Importantly, it possesses many of the regulatory components known to mediate cell growth and differentiation in C. crescentus. This common design makes it relatively straightforward to obtain a general understanding of the system, thereby greatly facilitating studies of its striking distinctive features. Having established a comprehensive toolbox for the genetic manipulation of H. neptunium (Jung et al., 2015), we have now started to study the mechanism of budding, a growth mode that is common among bacteria but still poorly understood. Another intriguing questions concerns the way in which the replicated chromosome is transported through the stalk structure that connects the mother cell body with the bud cell compartment. Preliminary data suggest a novel two-step process reminiscent of eukaryotic mitosis, in which chromosomal DNA is first fully replicated and segregated within the mother cell, followed by rapid translocation of one sister chromosome into the nascent bud (Jung et al., 2019). Finally, we are currently analyzing the mechanisms that control the highly asymmetric positioning of the divisome at the stalk-bud junction, a process that does not involve any of the regulatory systems known so far.
|
Chromosome segregation in H. neptunium. Shown is an S-phase cell carrying fluorescent labels attached to two origin-proximal loci.
|